Mass Percent of Sand in Unknown Mixture
Mass percent 2015881801528 x 100 011189 x 100 1118. What was the percent by mass of each component of the mixture.

Answered 1 Calculate The Mass Percent Of Sand Bartleby
A mixture of NaCl and NaBr has a mass of 208 g and is found to contain 076 g of Na.

. Mass percent mass of component total mass x 100. Calculations Total mass of original mixture- 102g Mass of sand. Name one heterogeneous mixture present in this lab.
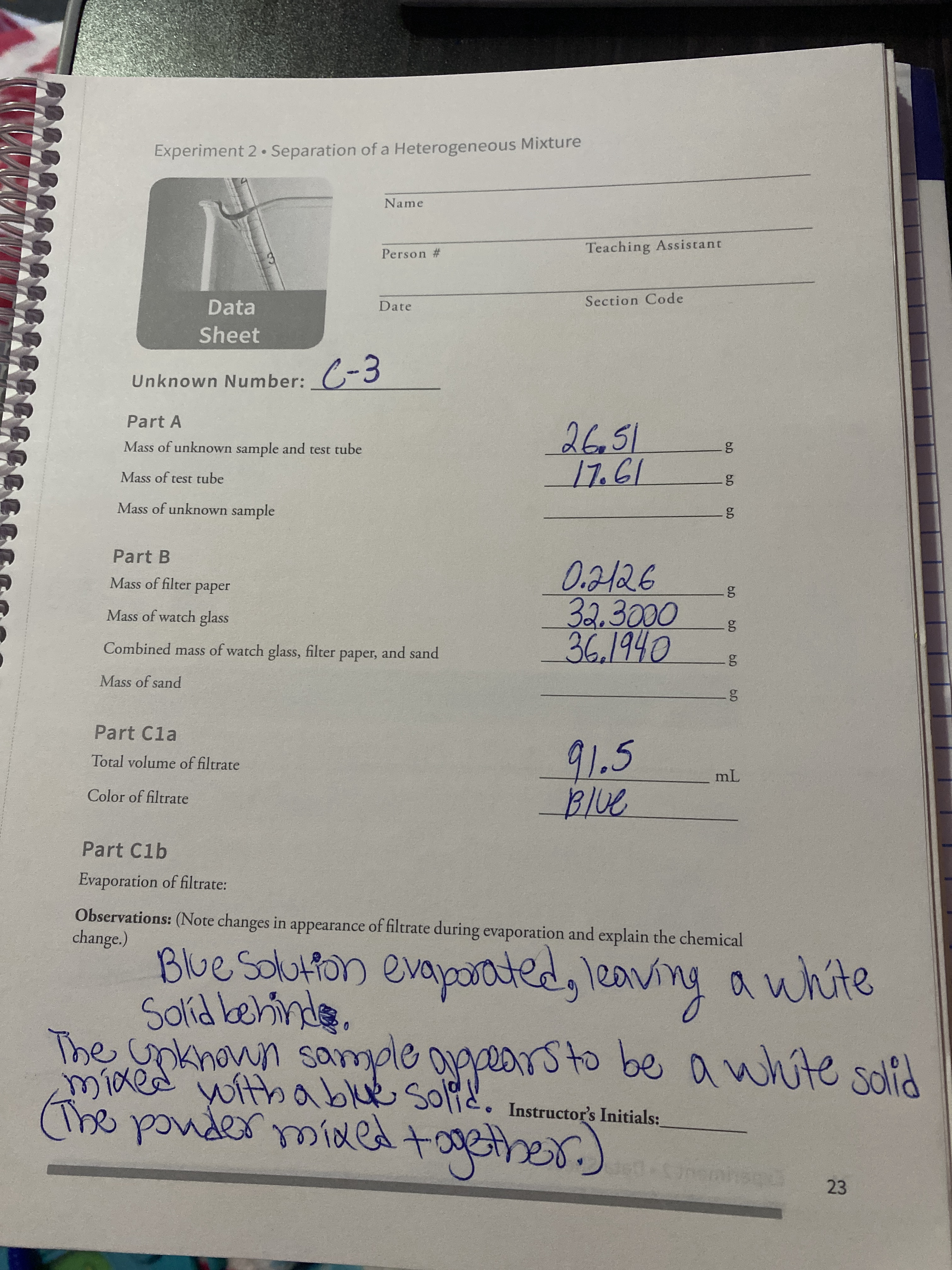
Yes Part A Data 846 Unknown Number Mass of test tube g Mass of unknown sample and test tube 9 1727 2555 Part B Data Mass of filter paper 9 Mass of watch glass g Combined mass of watch glass filter paper and sand 9 02187 302377 351182 Part C Data Total volume of filtrate mL 115 1pts Part A Calculations Mass of unknown sample. To determine the percentage of sand divide the weight of the sand by the weight of the combined salt and sand and multiple by 100. Once we know the mass of each component we can report its percentage.
Im doing some chemistry homework and a the problem in question goes as follows. Percent is a common measurement and its mathematical form is always the same. Mass of unknown sample and test tube 2683g.
The sum of all the mass percentages should add up to 100. As an aside we could have separated the sand from our sand-water mixture through evaporation alone just by letting it sit out in the open air. If you have 1015 grams of sand that you separated from 3010 grams of sandsalt mixture you.
The units of mass are typically grams. What is the mass of NaBr in the mixture. Calculate the mass of unknown sample.
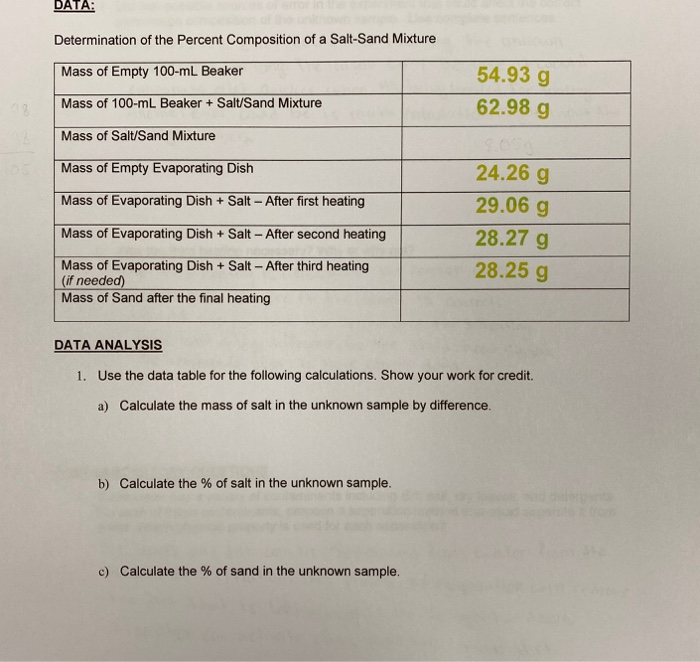
A student performs an experiment to determine the percentage by mass of M g C l X 2 in a 294 g sample of a mixture containing anhydrous M g C l X 2 and K N O X 3. Determine the mass of sand and the percent by mass of sand in your original unknown. For example if elemental analysis tells us that a potassium supplement contains 22 K by mass and we know that the K is present as KCl we can calculate the grams of KCl in the supplement.
Homework Equations Molar mass equations possibly formula mass The Attempt at a Solution Ive calculated the percent composition of Na in both NaCl and NaBr to be 3622 and 6378 respectively. Calculate the mass of copper II sulfate CUSO4. Subtracting the mass of the ammonium chloride and the mass of the sand from the mass of the original mixture gives the mass of the sodium chloride.
The mixture contained iron filings sand and salt. MCuSO4 is the mass of copper sulphate in your unknown. X100 whole part percent.
To get the total mass of the salt the mass of the evaporated salt should be multiplied by 5. This will give you the mass percent of the element. The sum of the mass percents of all components in a mixture should equal 100 the total mixture.
Sample in Sand Sample of Mass Sand of Mass 100 2. If you dont have the number of moles you will need to find the mass of copper sulphat. Since the portion of filtrate evaporated represents only 15 of the total it should contain only 15 of the salt that was originally dissolved.
Use Excel to make a pie chart of the percent composition of the mixture. Percentage of sand 120 g 120 g 150 g 080 g 100 30. Mass percent mass of solute mass of solution x 100.
We can control this by dividing the calculated mass of salt 407g by the total mass of the mixture 407 g of salt 752g of mixture X 100 541 salt An unknow sample has 459 sand and 541 salt. During this article well learn the mass percent formula with various solved numerical. Up to 24 cash back Subtract this number and the mass of the salt from the original total mass will help you find the mass of the sand.
Each component will need to be isolated and then weighed in order to calculate its mass percent accurately. Ask your instructor for the correct percent sand take your report sheet. Now that the equation is filled in simply solve to calculate the mass percent.
The number of solutes is expressed in mass or by moles. Mass percent mass component mass total mixture 100 Eqn. Divide the mass of the element by the total mass of the compound and multiply by 100.
065 g Mass of salt- 0233g Mass of calcium carbonate. Percentage of mass solutes mass mass of solution x 100. Elemental analysis can be used to determine the amounts of substances in a mixture.
Calculate the percent composition of sand in the mixture using the mass of the recovered sandCalculate the theoretical mass of salt in the mixtue using the mass of the recovered sandCalculate the theoretial percent composition of salt in the mixtureCalculate the percent error for the recovered saltCalculate the total mass of the recovered solids Weight of mixture. Assuming all the ceKClO3 decomposed to ceKCl and ceO2 calculate the mass percent of ceKClO3 in the original mixture I took the amount of ceO2 converted it to moles ceO2 then divided it to moles of just O then divived by 3 bcause for every 3 O for the 3 in the ceKClO3 there is a mole of ceKClO3 so. Calculate the percentage by weight of sand in the sample.
Mass of test tube 1780g. The student decides to precipitate all of the chloride ion C l X as A g C l by adding excess. However this would have taken a lot more time than filtering and then oven drying the sand.
Find the a Percent of sand in unknown mixture and the b Percent of copper sulfate in unknown mixture. Determining Mixture Percentages To determine the percentage of sand divide the weight of the sand by the weight of the combined salt and sand and multiple by 100. The mass percent of a component is defined by Eqn.
Answer Correct Sand From Instructor 5. Calculate the mass of sand. 0233g102g 228 sand.
065g102g 637 calcium carbonate. Where msample is the total mass of the sample. Mass of Unknown sample 903g.
Calculate the percent salt in the mixture. Calculating the mass of a substance in a mixture. Add the sand and salt weights.
Percentage of sand Mass of sand Mass of mixture 100 Explanation. If you have 1015 grams of sand that you separated from 3010 grams of sandsalt mixture you. Mass percent components mass total mass x 100 or.
Mass of watch glass 2823 g. Mass of filter paper 22 g. It shows the mass of solute present during a given mass of solution.
Calculate the mass percent. Mass percent is also known as percent by weight or ww. Once you know the mass of copper sulphate you can calculate the mass percentage as follows.
Calculate the mass of salt that must have been in the mixture. It is calculated as the mass of the component divided by the total mass of the mixture and then multiplied by 100 to get the percent. By mass CuSO4 100 mCuSO4msample.
The molar mass is the sum of the masses of all the atoms in one mole of the compound.
Answered 1 Calculate The Mass Percent Of Sand Bartleby
Solved Data Determination Of The Percent Composition Of A Chegg Com
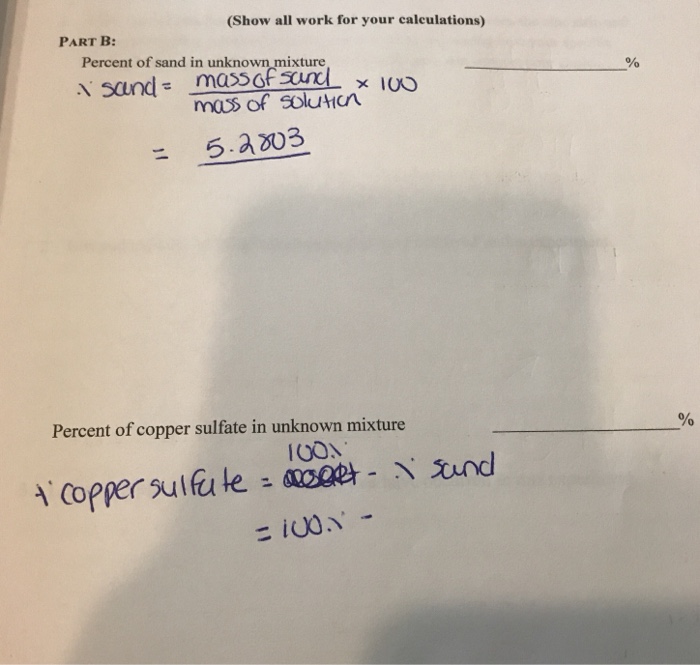
Comments
Post a Comment